top of page

THE HOODIN BLOG
Your source for AI, quality, and compliance updates, insights and best practices across the life sciences industry.
Global Reg Top Picks & Index by Hoodin – Week of October 14th, 2024
During the week of October 14th, the Global Index powered by Hoodin recognised 91 regulatory news and updates related to medical...

Team Hoodin
Oct 21, 20245 min read


Hoodin Partners with MedoQi to Deliver Cutting-Edge Regulatory Solutions to the Asia-Pacific Region
At Hoodin, we are delighted to announce an exciting new chapter in our journey towards revolutionizing regulatory compliance. We’ve...

Team Hoodin
Oct 17, 20242 min read


Global Reg Top Picks & Index – Week of October 7th, 2024
Cut through the noise of regulatory updates and stay ahead with the most impactful news from around the globe—handpicked and analysed!

Team Hoodin
Oct 14, 20245 min read


From Data to Insights: Transforming Regulatory Information into Actionable Intelligence
Watch our RAQA Deep Dive session featuring Nicholas Wells, an expert in regulatory strategy and intelligence, as he shares actionable...

Team Hoodin
Oct 11, 20244 min read


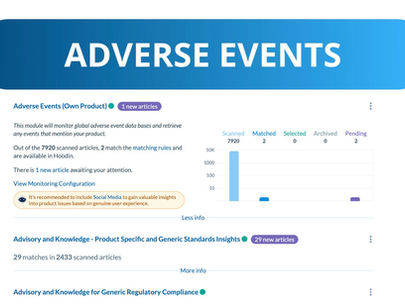
How to Simplify Complex Adverse Event Monitoring
Navigating the complexities of adverse event monitoring can be challenging. With data coming from diverse sources—like regulatory...

Team Hoodin
Sep 13, 20242 min read

The Webinar Gallery: The Comprehensive Resource for Your Professional Growth
We are thrilled to announce that all of our webinars from the past year have finally found a home! Our Webinar Gallery is now live,...

Team Hoodin
Aug 28, 20242 min read


How to Automate Reporting for Your Surveillance and Vigilance Activities
In an era where efficiency and precision are paramount, effective reporting is essential. Did you know that automating the reporting,...

Team Hoodin
Aug 21, 20242 min read


Catch Me If You Can: The Hide 'n Seek of Biocompatibility
Welcome to the Hoodin RAQA Deep Dive Sessions recordings and resources page! In this edition, we'll explore insights from our webinar:...

Team Hoodin
Aug 16, 20246 min read


Catch Me If You Can: The Hide 'n Seek of Biocompatibility – Join The Webinar Today!
The landscape of medical device regulations and standards is evolving rapidly, with an increased emphasis on surveillance and vigilance....

Team Hoodin
Aug 15, 20242 min read


How AI Enhances Your Surveillance & Vigilance Efforts: A Deep Dive into Hoodin's Integration
Artificial Intelligence (AI) has become an integral part of our daily lives, influencing everything from the way we communicate to how we...

Team Hoodin
Aug 14, 20242 min read


Enhancing Medical Device Safety: A Dive into Biocompatibility
Hope you're having a great summer! 🌞 As the landscape of medical device regulations continues to evolve, so does the emphasis on...

Team Hoodin
Jul 23, 20242 min read


Top Must-Read Books for RA and QA Professionals During Your Vacation
Finding time to relax and recharge is essential. Reading can be a great way to unwind while also enriching your professional knowledge. We asked 20 of our RAQA friends for tips on books to read during their vacations. Let us present the top 5 mentioned books and another 5 as additional picks. Whether you’re on a sunny beach or in a winter snow land , these books offer valuable insights that can enhance your professional life in RA and QA. Enjoy your reading, and with Hoodin b

Team Hoodin
Jun 28, 20243 min read


First Episode in New Series: The Power of Surveillance Hubs
We're thrilled to share our brand new series designed to provide you with the latest features, tools, and insights in the realm of surveilla

Team Hoodin
Jun 24, 20242 min read


The Power of Social Media for Vigilance and Adverse Event Monitoring
The Power of Social Media for Vigilance and Adverse Event Monitoring

Team Hoodin
Jun 14, 20243 min read


Top 5 Podcasts for RAQA Professionals in Life Sciences
The landscape of Regulatory Affairs and Quality Assurance (RAQA) in life sciences is complex, fast-evolving, and crucial for maintaining...

Team Hoodin
Jun 11, 20242 min read


Top Post-Market Surveillance Resources: Your Go-To Guide for Comprehensive Insights
Post-Market Surveillance (PMS) is a critical aspect of ensuring the safety and efficacy of medical devices after they are placed on the...

Team Hoodin
May 31, 20242 min read


AI and Smart Tools for Enhanced Surveillance
Welcome to the Hoodin RAQA Deep Dive Sessions post! In this edition, we'll explore insights from our recent special edition webinar where...

Team Hoodin
May 17, 20243 min read


The Power of AI in Regulatory Affairs
In today's rapidly accelerating intelligence and information landscape, the role of Artificial Intelligence (AI) in regulatory affairs is...

Team Hoodin
May 14, 20243 min read


Step Into the Future of Surveillance with the New Hoodin Version
Attention all industry professionals, the moment has finally arrived! After months of anticipation and hard work behind the scenes, we are

Team Hoodin
May 10, 20242 min read


RAQA Deep Dives with Veronika Valdova
Welcome to the Hoodin RAQA Deep Dive Sessions blog post! In this edition, we'll explore insights from our recent session with Veronika...

Team Hoodin
May 3, 20242 min read


bottom of page
