Request a Trial
Hoodin is specifically designed to perform monitoring for a life science product throughout their entire life cycle. With Hoodin, you have the flexibility to set up monitoring for a specific product (brand name) or a product type (such as a device, IVD product, or drug.) The trial project you will have access to will be customized to monitor the product or product type of your preference.
You will get access to a 14- day free trial of Hoodin
Request a Trial
Hoodin is specifically designed to perform monitoring for a life science product throughout their entire life cycle. With Hoodin, you have the flexibility to set up monitoring for a specific product (brand name) or a product type (such as a device, IVD product, or drug.) The trial project you will have access to will be customized to monitor the product or product type of your preference.
You will get access to a 14- day free trial of Hoodin
Request a Trial
Hoodin is specifically designed to perform monitoring for a life science product throughout their entire life cycle. With Hoodin, you have the flexibility to set up monitoring for a specific product (brand name) or a product type (such as a device, IVD product, or drug.) The trial project you will have access to will be customized to monitor the product or product type of your preference.
You will get access to a 14- day free trial of Hoodin
360 Surveillance &Vigilance
For Pharmaceuticals
Are you a pharmaceutical company striving for unwavering compliance in the ever-evolving landscape of EU and FDA regulations? Your quest for compliance and vigilance finds its solution in Hoodin.
1
Streamlined EU, FDA and local Compliance
Hoodin simplifies the intricate journey of pharmaceutical compliance in the EU, FDA regulations and regulations on any local market. Navigate the regulatory maze with ease and stay up to date with Hoodin.
Real-time Pharmacovigilance
Hoodin's robust monitoring tools keep you ahead by tracking adverse events in real-time, ensuring timely and appropriate responses in line with regulatory requirements.
2
3
Access to the Latest Literature
Empower your pharmaceutical endeavors with Hoodin's platform, providing instant access to the latest literature. Keep your pharmaceutical products grounded in the latest research and data.
Competitive Edge
Gain a competitive advantage with Hoodin's market monitoring tools, providing valuable insights into your industry landscape, and helping you adapt to changes in real-time, no matter where you are in the product lifecycle.
4
5
Continuous Regulatory Confidence
Trust Hoodin to keep you informed about evolving regulations, policies, and standards. Stay compliant at every stage with Hoodin's versatile support for your compliance needs.
Key features
and unique values
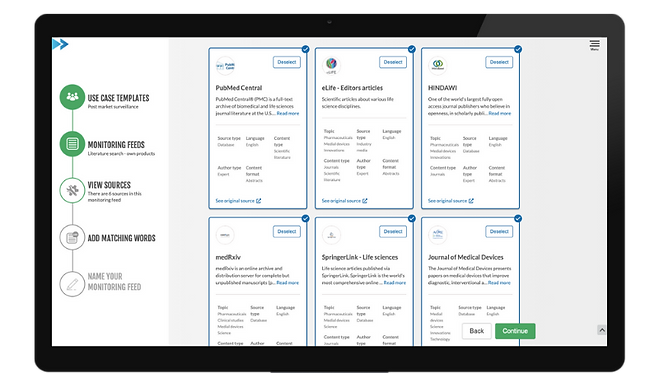
Monitor the most important and relevant source related to your need.
Monitor both own and competitor products.
Export results and insights for use in reports.
Share results and insights with invited peers (free feature).
Keep track of all changes made in your Hoodin project with the included change-log feature.
Validated by industry experts.

Experience 360 Surveillance & Vigilance!
Unlock the future of Medtech monitoring with Hoodin – where simplicity meets precision.
Effortless Trial
In just 2-3 hours, grasp the power of Hoodin. Our AI engines will craft a personalised Hoodin project, tailored to your unique medical device. Experience Hoodin today for 360 Surveillance & Vigilance.

High Value, Low Effort
The Hoodin platform ensures maximum value with minimal effort.
After your 14-day trial, you can choose to meet our experts for a detailed evaluation and validation of your tailored Hoodin project.

Experience Hoodin today – your gateway to streamlined, efficient Pharma monitoring with 360 Surveillance & Vigilance.